The heat capacity of hydrogen gas tells us a simple but critical piece of information: how much energy it takes to make it hotter. When you look at hydrogen, one thing stands out immediately—it heats up and cools down much faster than other common fuels. This quick thermal response is a double-edged sword, and it’s something you have to master to handle hydrogen effectively.
What Is Heat Capacity and Why It Matters for Hydrogen
Let's break down heat capacity with a simple kitchen analogy. Imagine you put a thin frying pan and a heavy cast-iron skillet on two identical burners. The thin pan gets screaming hot in seconds; it has a low heat capacity. The heavy skillet, on the other hand, takes a lot longer to heat up because it has a high heat capacity—it can absorb a lot more energy before its temperature climbs.

Hydrogen gas is the thin frying pan in this scenario. Its heat capacity is surprisingly low compared to traditional fuels. To put a number on it, the molar heat capacity of H₂ at constant pressure (Cp) is 28.836 J/mol·K under standard conditions. Compare that to methane (the main component of natural gas), which has a Cp of around 35.7 J/mol·K. This difference means it takes significantly less energy to raise the temperature of hydrogen. You can find more comparative fuel data from resources like the U.S. Energy Information Administration.
The Two Key Measures of Heat Capacity
When we talk about gases, "heat capacity" isn't a single, one-size-fits-all value. The conditions matter. That's why we use two distinct measurements:
- Cp (Constant Pressure): This is the heat required to raise the gas's temperature while letting it expand, so the pressure stays the same. Think of gas flowing through a pipeline or being used in an engine—this is the number you'd use.
- Cv (Constant Volume): This measures the heat needed when the gas is confined to a fixed space, like a sealed storage cylinder. As you add heat, the pressure builds, but the volume doesn't change.
Understanding the difference between Cp and Cv isn't just for textbooks; it's fundamental to designing safe storage vessels, managing pressurized pipelines, and predicting how hydrogen will behave under real-world operational loads.
This single property—heat capacity—has a ripple effect on everything. It dictates how fast a generator can be brought online, influences the materials we choose for pipelines to avoid thermal stress, and shapes the safety protocols for handling gas expansion. If you're working with mobile or temporary gas systems, getting a solid handle on this concept is non-negotiable for both efficiency and safety.
Understanding Constant Pressure (Cp) vs. Constant Volume (Cv)
When we talk about the heat capacity of hydrogen, the conversation always involves two key terms: Cp and Cv. The conditions under which you're heating the gas make all the difference, and understanding this distinction is crucial for anyone working with hydrogen in the real world.
Let's break it down with a simple analogy.
Imagine you have hydrogen in a completely sealed, rigid steel tank. The volume is fixed. As you pump heat into it, the gas molecules get energized, and the temperature rises. Since the gas can't expand, the pressure inside the tank climbs. This scenario represents heating at a constant volume, and the heat capacity here is called Cv.
Now, picture that same hydrogen in a cylinder with a movable piston on top, kind of like a syringe. As you add heat, the gas still gets hotter, but it also expands and pushes the piston up. This expansion does work. In this setup, the pressure can remain steady while the volume changes. This is heating at constant pressure, and its heat capacity is Cp.
Why Cp is Always Higher Than Cv
You'll notice in any data table that for hydrogen (or any gas, for that matter), Cp is always a bigger number than Cv. There's a very practical reason for this.
When you're heating the gas at constant pressure (the cylinder with the piston), the energy you're adding has to do two jobs:
- Raise the temperature: Just like in the sealed tank, it increases the internal energy of the gas, making the molecules move faster.
- Do expansion work: It also has to provide the energy needed for the gas to push that piston up and expand.
Because the energy you add for Cp has to cover both the temperature increase and the work of expansion, you have to put more energy in to get the same one-degree temperature change. It's as simple as that.
In short, Cv is purely about the energy needed to raise the temperature. Cp includes that same energy plus the extra energy the gas needs to expand against its surroundings.
This isn't just academic. For an engineer designing a pipeline where gas flows under steady pressure, using Cp is non-negotiable for thermal calculations. But for someone assessing how much the pressure will rise in a sealed storage tank left in the sun, Cv is the value that matters. Getting it wrong can lead to serious miscalculations in energy requirements, thermal management, and safety protocols.
How Temperature and Pressure Alter Hydrogen's Heat Capacity
The heat capacity of hydrogen gas isn't a single, fixed number. It's a dynamic property that shifts quite a bit with temperature and, to a lesser extent, pressure. For anyone designing systems that handle hydrogen—from high-pressure storage tanks to industrial heaters—getting a feel for this behavior is critical for both safety and efficiency.
Imagine a hydrogen molecule (H₂) at very low temperatures. It acts like a simple billiard ball, storing energy just by moving around. Scientists call this translational energy. When you add heat, its heat capacity stays pretty flat because this is the only way it can soak up the energy.
But as you warm things up, the molecule starts to do more. It begins to spin like a tiny dumbbell, opening up a new way to store energy called rotational energy. Once these rotational modes "unlock," you see a noticeable jump in the heat capacity.
The Role of Temperature in Energy Storage
At room temperature, hydrogen molecules are already zipping around and spinning freely. If you keep cranking up the heat, eventually you hit a point where the molecule itself starts to vibrate. The bond holding the two hydrogen atoms together begins to stretch and compress like a microscopic spring.
Activating this vibrational energy takes a lot more heat. But once it kicks in, it provides yet another "storage locker" for thermal energy, causing the heat capacity of hydrogen gas to climb once again.
- Low Temperatures: Only translational motion is active.
- Moderate Temperatures: Translational and rotational motions are both active, increasing heat capacity.
- High Temperatures: Vibrational motion finally begins, causing another step-up in heat capacity.
At extreme temperatures, the story continues. The temperature-dependent surge in hydrogen gas's specific heat capacity reveals its unique behavior under industrial heat loads. Engineering data shows Cp for H₂ climbs steadily from 14.65 J/mol·K at 750 K to 20.96 J/mol·K at 6000 K, a jump of over 43%, partly due to molecular dissociation. You can explore more detailed thermal property data on resources like the Engineering ToolBox.
How Pressure Changes the Game
While temperature is the main character in this story, pressure also plays an important role, especially in the real world of compressed gas delivery. At low pressures, hydrogen behaves predictably, almost like a textbook "ideal gas." But once you squeeze it into a high-pressure tank, things get more complicated.
At high pressures, the molecules are crammed together. The subtle forces between them, which you can safely ignore at atmospheric pressure, suddenly start to matter. This "real gas" behavior can alter the heat capacity, and engineers have to account for these deviations when designing storage vessels or regulating gas flow.
The infographic below shows the fundamental difference in how hydrogen absorbs heat when its volume is fixed versus when its pressure is fixed.
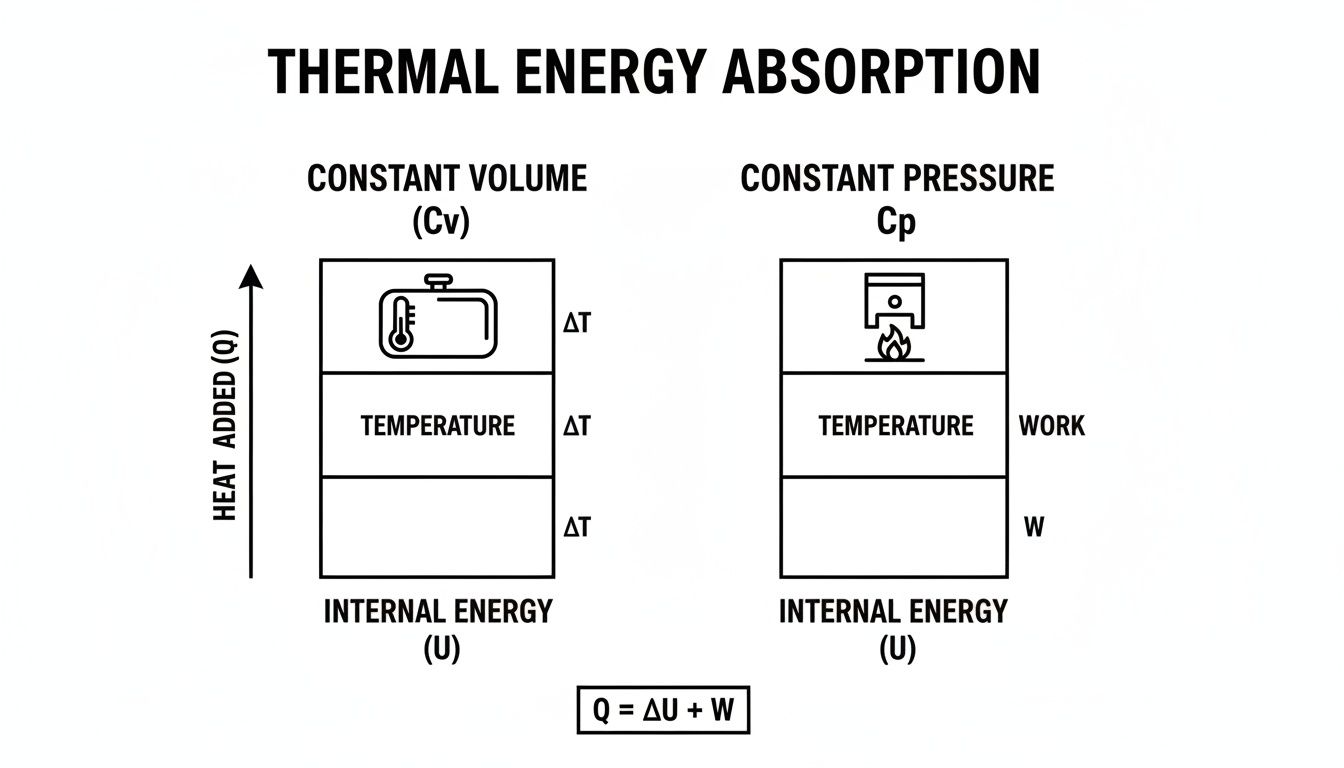
This visual gets to the heart of it: in a constant pressure system, like a pipeline, the energy you put in has to do two jobs—raise the temperature and do work by letting the gas expand.
Hydrogen vs. Natural Gas: A Practical Heat Capacity Comparison
When you're choosing an industrial fuel, the numbers tell a story—one of efficiency, speed, and ultimately, operational cost. Pitting the heat capacity of hydrogen gas against natural gas (which is mostly methane) reveals some stark differences, and these differences have major consequences in the real world, especially for mobile and temporary energy projects.
Think back to our analogy of the frying pan versus the heavy skillet. Hydrogen is the lightweight, nimble pan that gets screaming hot almost instantly. Natural gas is the heavy, cast-iron skillet; it's steady, but it takes its sweet time to get up to temperature. This isn't just a quirky characteristic; it’s a core operational advantage for hydrogen in the right situations.
Why Speed Matters in Industrial Heating
For any job that requires fast startups or precise temperature cycling, hydrogen’s low heat capacity is a game-changer. Imagine firing up a new power generator or a massive industrial furnace. Every minute spent waiting for that equipment to reach its operating temperature is downtime, and downtime costs money.
Because hydrogen heats up and cools down so quickly, processes can start faster, cycle more efficiently, and shut down with more control. This thermal responsiveness is exactly why it's becoming the fuel of choice for high-stakes projects where every second on the clock matters.
The efficiency advantage is clear. Comparing hydrogen's heat capacity to natural gas highlights its superior thermal response. Hydrogen's room-temperature specific heat is far lower than the range for natural gas, granting H₂ a 60-70% lower energy requirement for temperature swings. This is ideal for quick-start scenarios where units must be deployed and operational in hours, not days. You can find more data behind this comparison on Wikipedia).
To really see the difference, it helps to put the numbers side-by-side.
Heat Capacity Face-Off: Hydrogen vs. Natural Gas (Methane)
Here’s a direct comparison of the key thermal properties that define how these two fuels behave in industrial settings.
| Property | Hydrogen (H₂) | Natural Gas (CH₄) | Key Implication |
|---|---|---|---|
| Molar Cp (J/mol·K) | 28.8 | 35.7 | Hydrogen requires ~20% less energy to heat per mole, enabling faster thermal cycling and quicker startups. |
| Mass Cp (J/g·K) | 14.3 | 2.2 | Per gram, hydrogen can absorb far more energy, making it an excellent coolant where weight is a factor. |
| Response to Heat | Rapid temperature change | Slower, more gradual change | Ideal for applications needing precise, quick temperature adjustments, reducing costly operational delays. |
This table shows two different stories. On a per-mole basis (Molar Cp), hydrogen is nimbler and heats up faster. But on a per-gram basis (Mass Cp), its ability to soak up energy is off the charts, making it an incredible coolant.
Practical Implications for Mobile Gas Delivery
For companies like Blue Gas Express that deliver mobile energy solutions, these properties translate directly into real-world benefits for customers. Whether a construction site needs temporary heat to meet an occupancy permit deadline or a utility needs to commission an emergency generator yesterday, speed is everything.
Hydrogen's low heat capacity ensures that fuel can bring systems online far quicker than natural gas. This means less fuel is burned just during warmup, project timelines are met more reliably, and the entire temporary energy setup runs more efficiently. It’s this kind of thermal performance that makes hydrogen such a powerful tool for today's on-demand industrial energy needs.
Putting Heat Capacity to Work in the Real World
Knowing the theory behind hydrogen's heat capacity is great, but its real value comes alive when you apply it to solve everyday engineering problems. Let's move from the textbook to the field and see how these numbers drive critical decisions about safety, cost, and efficiency.
Whether you're sizing a heater or budgeting for a project, the core calculation is refreshingly simple. It all boils down to one fundamental formula:
Total Energy (Q) = Mass (m) × Specific Heat Capacity (Cp) × Change in Temperature (ΔT)
This little equation is your key to figuring out exactly how much energy it takes to get hydrogen from one temperature to another.

A Practical Example: Heating for a Process Startup
Let’s imagine a common scenario. An industrial furnace needs a batch of hydrogen, but it has to be heated from its storage temperature before it can be used.
- The Goal: Heat 10 kg of hydrogen gas.
- The Conditions: Starting at 15°C, we need to get it to 200°C.
First, we need the specific heat capacity (Cp) for hydrogen, which is a hefty 14.3 J/g·K (or 14,300 J/kg·K). Now we just plug in the numbers.
Find the Temperature Difference (ΔT):
- ΔT = Final Temperature – Initial Temperature
- ΔT = 200°C – 15°C = 185°C. (Since a 1°C change is the same as a 1 Kelvin change, our ΔT is also 185 K).
Calculate the Required Energy (Q):
- Q = 10 kg × 14,300 J/kg·K × 185 K
- Q = 26,455,000 Joules
That's a lot of zeros, so we'll convert it to something more manageable: 26.45 Megajoules (MJ).
Knowing you need 26.45 MJ is incredibly powerful. This single number informs what size heater to install, how much fuel you'll burn to get the job done, and how long the startup process will take.
This simple calculation is the bedrock of thermal management. Getting the energy prediction right means you don’t waste money on oversized equipment or suffer performance issues with an undersized system. It’s about matching the tool to the task perfectly.
Beyond Heaters: Managing Pipeline Temperatures
Heat capacity isn't just for planned heating. It's also vital for managing the complex thermal behavior of hydrogen as it moves through pipelines.
When hydrogen flows, pressure changes can cause it to heat up or cool down unexpectedly due to thermodynamic effects. Engineers rely on heat capacity data to model these temperature swings. Why? Because if the gas gets too cold, the steel pipe can become brittle and fail. If it gets too hot, it could compromise the pipeline's structural integrity.
By calculating heat loss to the ground over miles of pipe, engineers can determine if they need to add insulation or even supplemental heating to keep the gas within a safe temperature window.
Ultimately, a solid grasp of hydrogen's thermal properties is non-negotiable for safe and efficient system design. From massive industrial furnaces to the stability of a regional gas grid, these calculations transform physics into practical decisions that keep operations running smoothly and, most importantly, safely.
Key Safety Considerations for Hydrogen Handling
Hydrogen’s unique thermal properties are what make it so effective, but they also mean we need to be incredibly disciplined when it comes to safety. Because the heat capacity of hydrogen gas is so low, its temperature can change in the blink of an eye. While that's a huge advantage for getting systems up and running quickly, it puts immense thermal stress on the materials used for storage and transport, which can become brittle and fail if not chosen correctly.
This rapid temperature swing is most apparent during depressurization, a process governed by what's known as the Joule-Thomson effect. Imagine opening a valve on a high-pressure tank. As the hydrogen rushes out and expands, its temperature can drop dramatically. This sudden, intense cooling can make standard steel valves and fittings dangerously brittle, creating a serious risk if the equipment isn't specifically rated for cryogenic-like conditions.
On the flip side, compressing hydrogen quickly does the exact opposite—it generates a lot of heat. This is a critical reason why managing all potential heat sources anywhere near hydrogen fuel is absolutely non-negotiable.
Managing Thermal Risks in the Field
Building a solid safety plan starts with understanding and planning for these thermal behaviors. Since hydrogen is in a class of its own, following strict protocols is essential. Many of the core principles found in guides for the safe handling of hazardous materials apply here, as disciplined procedures are universal.
Here are a few of the most important protocols:
- Material Compatibility: Always, always use materials specifically designed and rated for hydrogen service. This is especially true for components in high-pressure systems that might experience extreme temperature drops.
- Controlled Venting: Never vent high-pressure hydrogen quickly. Your procedures must be built around slow, controlled depressurization to keep system components from getting dangerously cold.
- Ignition Source Control: Be relentless about eliminating any and all potential ignition sources. This includes everything from sparks and static electricity to hot surfaces, especially during refueling or maintenance.
- Proper Ventilation: Make sure any area where hydrogen is stored or handled has more than enough ventilation. This prevents the gas from accumulating to dangerous levels if a leak ever occurs.
The bedrock of hydrogen safety is discipline. Every single step, from connecting a line to operating the system and disconnecting it, must follow a proven, tested procedure. There are simply no shortcuts when you're working with a fuel this powerful.
At the end of the day, understanding the heat capacity of hydrogen gas isn't just about designing an efficient system. It’s about building a fundamentally safe operating environment from the ground up.
Answering Common Questions About Hydrogen's Heat Capacity
Digging into the details of hydrogen's thermal properties often brings up some great questions. Let's tackle a few of the most common ones to help build a more practical understanding.
Why Is Hydrogen's Heat Capacity So Much Lower Than Other Gases?
It all comes down to its incredibly simple structure and low mass. Hydrogen is the lightest element in the universe, and its H₂ molecule is just two atoms bonded together. At normal temperatures, it mainly stores thermal energy through movement (translation) and rotation.
Now, think about a more complex molecule like methane (CH₄). It has more atoms and, therefore, more ways to vibrate and move. You can almost picture it like having more "storage lockers" for energy. Since hydrogen is so light and has fewer of these energy storage options, it doesn't take much energy to get its molecules moving faster—which is all temperature really is.
How Does Liquid Hydrogen's Heat Capacity Compare to Gaseous Hydrogen?
They are worlds apart. Liquid hydrogen (LH₂) has a much, much higher specific heat capacity than hydrogen gas, especially around its boiling point of -252.87 °C. This means the liquid can soak up a massive amount of heat before its own temperature starts to climb, which is why it's such an incredible cryogenic coolant.
But this property is also its biggest challenge. Because liquid hydrogen is so good at absorbing heat from its surroundings, it wants to boil off constantly. This is why it requires extremely sophisticated, vacuum-insulated containers just to keep it in its liquid state.
This stark difference is at the heart of cryogenic engineering. The gas is easy to heat up, while the liquid is fantastic at staying cold and absorbing heat. It's a duality that engineers have to manage very carefully in any system design.
Does the Ortho-Para Ratio Really Matter for Heat Capacity?
Absolutely, and it's a big deal, especially at very low temperatures. Hydrogen gas isn't just one thing; it's a mix of two different "spin isomers": orthohydrogen and parahydrogen. These two forms are based on the nuclear spin of their protons, and this difference gives them unique rotational energy levels and, consequently, different heat capacities.
At room temperature, the natural mix is stable at about 75% ortho and 25% para. But as you cool the gas down to liquefy it, the molecules start converting to the lower-energy para state. This conversion process actually releases a surprising amount of heat. If you don't actively manage this, that extra heat can cause your freshly liquefied hydrogen to literally boil away.
When your project demands a reliable, on-demand energy source, turn to the experts. Blue Gas Express delivers mobile natural gas solutions to keep your operations running without interruption. Learn more about our temporary CNG and LNG services.