Think of the massive, unseen network of pipelines buried beneath our cities and countryside. These are the arteries of our modern world, and they face a constant, silent enemy: corrosion. This natural process relentlessly attacks metal, threatening to weaken and destroy the vital infrastructure we depend on.
Enter cathodic protection, a brilliant electrochemical technique that acts as an invisible shield for these pipelines.
How Cathodic Protection Halts Corrosion in its Tracks
At its most basic, corrosion is just metal trying to return to its natural, stable state—like iron turning back into iron oxide, which we know as rust. It's an electrochemical reaction where tiny areas on a pipe's surface act as anodes (where corrosion happens) and cathodes (where it doesn't).
Cathodic protection cleverly short-circuits this natural process. It works by making the entire surface of the pipeline a cathode. By forcing the pipe into this protected state, we effectively stop the corrosive reaction before it can even start. It’s less like a coating that just covers the problem and more like a permanent cure that changes the pipe's electrical behavior.
The Science Made Simple
Imagine two different metals buried in the soil. One will naturally corrode faster to protect the other. Cathodic protection harnesses this very principle. We introduce a more "active" metal (an anode) nearby and connect it to the pipeline. This anode willingly sacrifices itself, corroding away while feeding a steady stream of protective electrons to the pipe.
This flow of current satisfies the pipe's natural tendency to lose electrons, essentially neutralizing the driving force behind rust and decay.

As you can see in the diagram, the external anode gives itself up to protect the much larger and more critical pipeline. This sacrificial system is a simple yet powerful way to divert corrosion away from the asset you need to preserve.
A Critical Investment in Safety and Reliability
So, why is this so important? Because pipeline failures are not just inconvenient; they can be catastrophic. A breach can lead to environmental disasters, costly service outages, and serious public safety risks.
By proactively managing the electrochemical process of corrosion, cathodic protection systems ensure that vital pipelines can operate safely and efficiently for decades, preventing costly leaks and service disruptions.
The global reliance on this technology speaks volumes. The cathodic protection market was recently valued at USD 5.25 billion and is on track to hit nearly USD 7.82 billion soon. This isn't just a niche industry; it's a fundamental part of maintaining the integrity of our global infrastructure. You can learn more about these market trends and their implications for pipeline integrity.
How Cathodic Protection Halts Corrosion
To get a handle on how cathodic protection works, you first have to understand the enemy: corrosion. It’s not just a simple stain on your pipes. Corrosion is an active electrochemical process, basically a tiny, unwanted battery chugging away on the surface of your pipeline. For this destructive little battery to get going, it needs four key things to form what we call a "corrosion cell."
Think about it. A buried steel pipe is never sitting in a perfectly consistent environment. Tiny differences in soil moisture, pH levels, oxygen, or even the metallurgy of the pipe itself create small electrical imbalances across its surface. And that’s all it takes to kickstart the whole corrosive mess.
This corrosion cell has four essential parts:
- The Anode: This is the trouble spot where corrosion actually happens. Metal at the anode gives up tiny particles (electrons) and literally dissolves into the soil. This is where your pipe rusts, pits, and eventually fails.
- The Cathode: This is the protected zone that accepts the electrons flowing from the anode. It doesn't corrode, but it’s a required partner for the reaction to happen.
- The Electrolyte: For a buried pipe, the electrolyte is just the moist soil. It’s any medium that can conduct electricity and allows the current to flow between the anode and cathode.
- The Metallic Path: This is the pipe itself. It provides the physical connection for electrons to travel from the anode site to the cathode site, completing the circuit.
When you have all four of these present, corrosion is going to happen. It's just nature. The iron in the steel pipe wants to give away its electrons at the anode, which is the very definition of rust. Cathodic protection is all about cleverly stepping in and breaking this destructive cycle.
Supplying a Better Alternative
The whole strategy behind cathodic protection is brilliantly simple. Instead of letting the pipe eat away at itself, we introduce a new, more attractive source for those electrons. By doing this, we turn the entire pipeline into a cathode—the protected part of the circuit—and stop anodes from ever forming on its surface.
The fundamental goal of cathodic protection is to overwhelm the natural corrosion currents leaving the pipe by supplying a stronger, protective current from an external source. This forces the entire pipe surface to become a net receiver of current, making corrosion impossible.
This protective current gives the pipe what it electrochemically "wants" without forcing it to sacrifice its own metal. We're essentially redirecting the corrosion away from our valuable pipeline and onto a separate, replaceable component that's designed to be destroyed. This is how cathodic protection for pipes provides a constant, active defense against failure.
This visual shows how a pipeline is protected using the two primary methods we're about to cover.
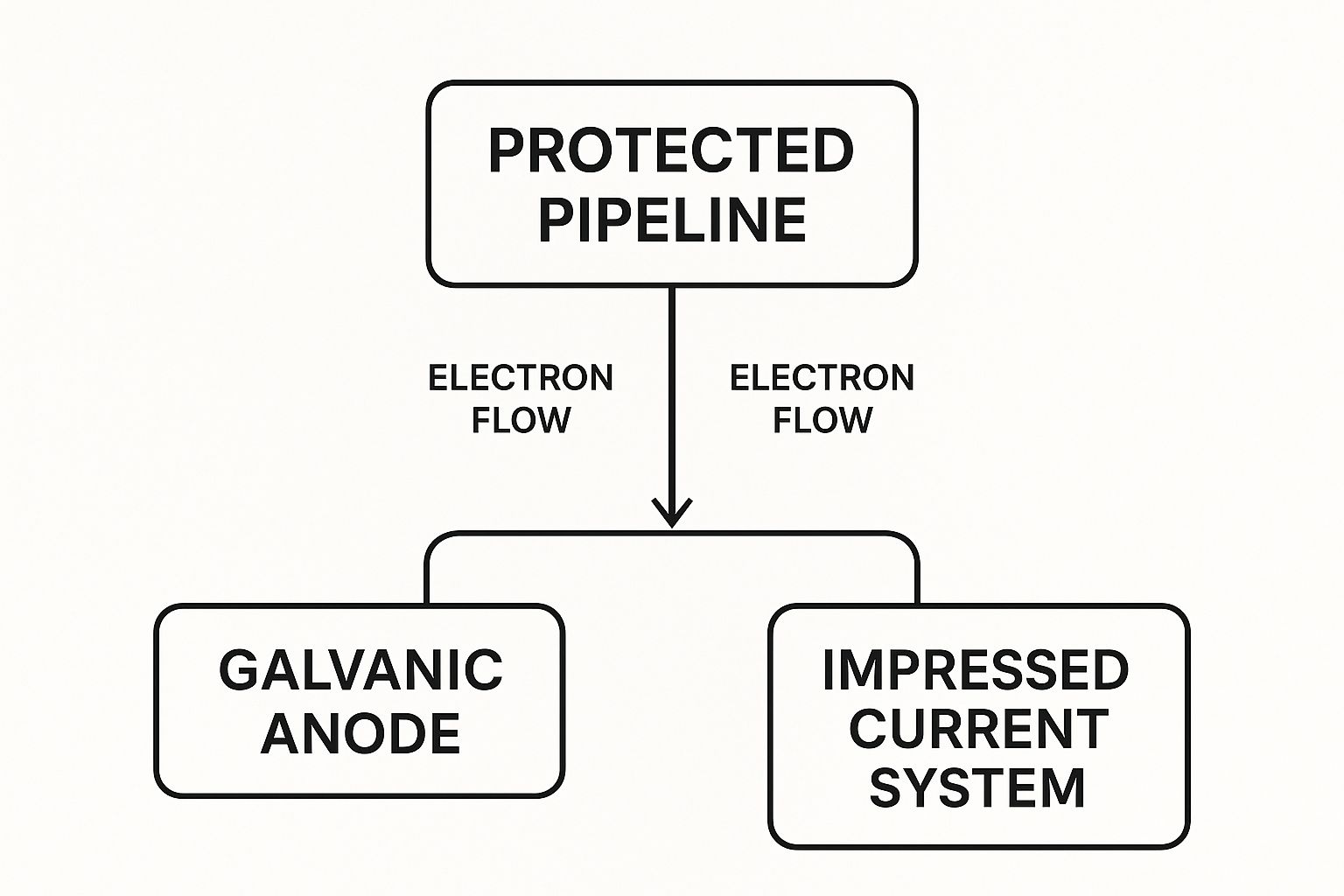
As you can see, both the Galvanic and Impressed Current systems achieve the same end goal—they supply protective electrons to the pipe. They just get there in different ways.
Introducing the Two Primary Strategies
In the field, we use two main techniques to apply this protective current and keep pipelines from turning to rust. Each has its pros and cons and is suited for different situations, but they both work on that same core principle of turning the pipe you want to save into a cathode.
The two main types of cathodic protection are:
- Galvanic Cathodic Protection: We often call this a "sacrificial" system. It works by connecting anodes made from a metal that's more electrochemically active than steel—think zinc, aluminum, or magnesium. These anodes naturally corrode, or "sacrifice" themselves, to protect the pipe. It's a simple, self-powered system.
- Impressed Current Cathodic Protection (ICCP): This is the more powerful approach. It uses an external power source, usually a rectifier, to "impress" a direct current onto the pipeline through a series of long-lasting anodes. We use this for big jobs, like long transmission pipelines or pipes with poor coatings.
The choice between these two methods comes down to a handful of key differences in how they operate, what they cost, and where they work best.
Comparing Galvanic vs Impressed Current Cathodic Protection
To make it clear, let's break down the two main strategies side-by-side. Think of the galvanic system as using a natural battery and the impressed current system as plugging one into the wall.
| Feature | Galvanic (Sacrificial) System | Impressed Current System |
|---|---|---|
| Power Source | Natural voltage difference between anode and pipe (no external power) | External DC power source (rectifier) |
| Anode Material | Active metals like zinc, aluminum, magnesium (they get consumed) | Inert materials like high-silicon cast iron, graphite (long-lasting) |
| Driving Voltage | Low (typically 0.5-1.5 volts) | High and adjustable (often 10-50 volts or more) |
| Current Output | Low and self-regulating | High and controllable |
| Typical Use Cases | Smaller structures, well-coated pipelines, isolated areas, hotspots | Large pipelines, bare or poorly coated pipes, high-resistivity soils |
| Installation & Maintenance | Simpler to install, requires periodic anode replacement | More complex installation, requires regular monitoring of the power source |
| Stray Current Risk | Very low risk to other structures | Higher risk of causing interference on nearby metallic structures |
In short, the decision isn't just about picking one over the other. It's about a careful engineering assessment of the pipeline's size, its coating quality, the corrosivity of the soil, and of course, the budget and long-term maintenance plan.
Choosing Your Method: Galvanic vs. Impressed Current

When it comes to picking the right kind of cathodic protection for your pipes, there’s no single "best" answer. The choice between a galvanic system and an impressed current system really boils down to the specific needs of your pipeline, the ground it sits in, and what you need to achieve long-term.
Think of it this way: are you looking for a simple, self-sufficient "set it and forget it" solution, or do you need a powerful, adjustable system that can protect a massive network? Both methods get the job done—they stop corrosion by making the pipe the "cathode" in an electrochemical cell—but they go about it in completely different ways. Getting this choice right is the first step to building a system that’s both effective and economical.
Galvanic Systems: The Sacrificial Shield
Galvanic cathodic protection is beautiful in its simplicity. It taps directly into the natural laws of electrochemistry by connecting a more reactive metal, known as a sacrificial anode, to the steel pipeline. These anodes are usually made from alloys of zinc, aluminum, or magnesium.
Because these metals are naturally more electrochemically active than steel, they have a stronger urge to corrode. When you connect one to a pipe and bury them both, the anode essentially "sacrifices" itself by giving up its electrons. This creates a small but steady DC current that flows to the pipeline, satisfying its electrical appetite and stopping it from rusting.
It’s like tossing a steak to a lion so it leaves you alone. The anode corrodes so the pipeline doesn't have to.
At its heart, a galvanic system is a self-contained, self-regulating battery. It needs no outside power, making it the perfect choice in situations where simplicity and reliability are the top priorities.
This straightforward approach makes it a fantastic fit for certain jobs.
Where Galvanic Systems Shine:
- Smaller or Isolated Pipelines: They're perfect for protecting shorter pipe runs, brand-new pipes with excellent coatings, or other isolated metal structures where a big system would be overkill.
- Areas Without Power: Since they generate their own current, galvanic systems are the default choice for remote locations where running power lines would be a nightmare.
- Hot Spot Protection: Got a specific, localized corrosion problem on an otherwise healthy pipeline? A few galvanic anodes can target that "hot spot" without the need for a full-blown system.
- Low-Risk Environments: The low voltage output of these systems makes them a much safer option in congested areas where you need to avoid causing electrical interference with other buried utilities.
Of course, that simplicity comes with trade-offs. The driving voltage is low and fixed, which means a galvanic system might not have enough oomph to protect a large, poorly coated pipeline or one buried in high-resistance soil.
Impressed Current Systems: The Powered Protector
When you need more muscle and a whole lot more control, an Impressed Current Cathodic Protection (ICCP) system is the way to go. Instead of relying on the natural voltage between two different metals, an ICCP system uses an external DC power source—called a rectifier—to actively "impress" a protective current onto the pipeline.
The rectifier takes standard AC power from the grid and converts it into a precisely controlled DC output. That current is then sent to a series of incredibly durable anodes buried nearby. These anodes, typically made from materials like high-silicon cast iron or mixed metal oxide, are built to last for decades, discharging current without being consumed like their sacrificial counterparts.
The real game-changer with ICCP is control. If soil conditions change or the pipe's coating degrades over the years, an operator can simply walk up to the rectifier and turn up the juice. This ensures the pipeline stays fully protected no matter what.
It’s this power and adaptability that makes ICCP the go-to for major infrastructure.
Key Advantages of Impressed Current Systems:
- High Current Output: They can pump out far more current than galvanic anodes, making them capable of protecting huge pipeline networks, even older ones that are bare or have damaged coatings.
- Adjustable and Controllable: You can fine-tune the protective current at any time to adapt to changing needs over the pipeline's entire lifespan.
- Long Anode Lifespan: ICCP anodes are designed for the long haul, often lasting 20 to 40 years or more, which means far less digging and replacement.
- Cost-Effective for Large Projects: When you're protecting miles and miles of pipe, the cost-per-mile for an ICCP system is often significantly lower than installing thousands of individual galvanic anodes.
This power makes them the standard for major oil, gas, and water transmission lines. But with great power comes greater complexity. They're more involved to design and install, need a constant supply of electricity, and, if not engineered correctly, can create stray currents that interfere with other nearby structures.
Direct Comparison: Galvanic vs. Impressed Current
Sometimes, seeing things side-by-side makes the choice much clearer. Here’s a quick breakdown of how the two methods stack up.
| Feature | Galvanic (Sacrificial) System | Impressed Current (ICCP) System |
|---|---|---|
| Power Source | Self-powered via natural electrochemistry | External AC/DC power source (rectifier) |
| Driving Voltage | Low & fixed (typically 0.5-1.5V) | High & adjustable (often 10-50V or more) |
| Anode Type | Consumable (zinc, magnesium, aluminum) | Non-consumable (high-silicon cast iron, MMO) |
| Maintenance Needs | Periodic anode replacement (every 10-20 years) | Regular checks on the rectifier and power supply |
| Initial Cost | Generally lower for small jobs | Higher due to rectifier, cabling, and power hookup |
| Operating Cost | None (self-powered) | Ongoing electricity costs |
| Best Application | Small, well-coated pipes; remote sites; hot spots | Large, long pipelines; poorly coated pipes; complex networks |
Ultimately, choosing the right cathodic protection for pipes isn't a guess—it's an engineering decision. A thorough analysis of factors like soil resistivity, coating quality, the sheer scale of the project, and your long-term operational plans will point you toward the right shield for your pipeline.
Designing a Cathodic Protection System
Putting an effective cathodic protection for pipes system in place is a whole lot more than just burying some metal and hooking up a few wires. It's a precise engineering discipline that begins long before a single shovel hits the dirt. A successful design is a custom-tailored plan, built from the ground up to match the specific pipeline and its unique environment.
Think of it like a doctor prescribing medicine. You wouldn't want a generic pill without a proper diagnosis, right? It's the same with CP. The system needs a detailed investigation to understand the "illness"—the specific corrosion risks—before the right "treatment" can be designed. This pre-design phase is where the success of the entire project is decided.
Initial Surveys: The Diagnostic Phase
Before an engineer can even start sketching out a design, they have to become a bit of a detective. The first job is to get out in the field and conduct a series of crucial surveys to gather data on the pipeline and its surroundings. This information is the bedrock upon which the entire protective system is built.
Key investigations include:
- Soil Resistivity Testing: This is arguably the most critical initial test. It tells us how easily an electrical current can travel through the soil. Low-resistivity soil, like wet clay, is a hotbed for corrosion and will demand more protective current. On the flip side, high-resistivity soil, like dry sand or rock, can make it tough for the current to get from the anode to the pipe.
- Coating Integrity Assessment: Let's be realistic: no pipe coating is perfect. Engineers have to figure out the quality of the existing coating to spot potential weak points, which we call "holidays." A pipeline with a shoddy coating is going to need a much beefier CP system to make up for all that exposed metal.
- Foreign Structure Surveys: What else is buried nearby? Other pipelines, storage tanks, or even utility lines can mess with a new cathodic protection system. Catching these potential "stray current" problems early on is essential to avoid accidentally causing corrosion on your neighbor's assets.
Gathering all this data gives us a complete picture of the challenges we're up against. Without it, you’re just flying blind.
Calculating Current Demand
With the survey data in hand, the next critical step is to calculate the current density demand. This is the exact amount of electrical current needed per square foot of exposed steel to bring corrosion to a dead stop. It’s the precise "dosage" of protection your pipeline needs.
This calculation isn't just plugging numbers into a simple formula; it's a mix of hard science and seasoned experience.
The goal is to apply enough current to shift the pipe's natural electrical potential to a protected level, typically a minimum of -850 millivolts relative to a copper-copper sulfate reference electrode. This industry-standard measurement is the definitive sign that the pipe has been successfully turned into a cathode.
The final number is influenced by everything we learned in the surveys—the soil conditions, the estimated number and size of coating holidays, and the pipeline's total surface area. A poorly coated pipe in aggressive soil might need 10-20 times more current than a well-coated one in friendly soil. This calculation is what tells us how big the anodes need to be, how many we'll need, and, for an impressed current system, how powerful the rectifier must be.
Strategic Anode Placement
Once we know how much current we need, the question becomes: how do we deliver it effectively? The placement of anodes—whether they're the sacrificial type or part of an impressed current system—is a strategic game that can make or break the whole setup. Place them poorly, and you’ll get patchy protection, leaving parts of your pipeline wide open to attack.
Engineers have to map out:
- Anode Distribution: Anodes must be spaced out properly along the pipeline to ensure the protective current blankets every square inch of the structure.
- Groundbed Design: For impressed current systems, the anodes are installed in what's called a "groundbed." The design of this groundbed heavily influences how well the current spreads out into the earth.
- Environmental Factors: Placement has to account for all those variations in soil resistivity we found earlier. We might need to cluster more anodes in areas with particularly aggressive soil.
The final design also pinpoints the locations for test stations. These are permanent access points built along the pipeline, allowing technicians to take regular measurements and monitor the system’s health for decades. They are the windows that let us ensure this invisible shield remains strong and effective year after year.
Monitoring and Maintaining Your System

Putting a cathodic protection system in the ground isn't the finish line. Far from it. It's the start of a long-term relationship with your pipeline's integrity. Think of it less as an installation and more as deploying a permanent guardian for your asset. But like any guardian, it needs regular check-ups to make sure it's awake, alert, and doing its job 24/7.
This ongoing oversight is what truly separates a protected pipe from one that’s just a sitting duck. Without consistent monitoring, even the most brilliantly designed system can slowly degrade, leaving your pipeline exposed to the exact corrosion you sought to prevent. It’s this diligence that ensures your initial investment pays off for decades to come.
Key Performance Indicators for System Health
So, how do we know if the system is actually working? It all comes down to measuring a few key electrical characteristics, and the most important one is the pipe-to-soil potential. This reading, taken at various test stations along the pipeline, is the definitive proof of whether your pipe is electrochemically safe or not.
The gold standard in the industry is a pipe-to-soil potential of -850 millivolts (mV) or more negative when measured with a copper-copper sulfate reference electrode. If that number creeps up and gets less negative than -850 mV, you've got a red flag. It means parts of your pipe could be actively corroding.
Beyond that critical number, the routine checks get more specific depending on the type of system you have.
- For Galvanic Systems: The main job here is to periodically check the current coming off the sacrificial anodes. If you see that output start to drop, it's a clear sign the anodes are being consumed and are nearing the end of their life. Time to schedule a replacement.
- For Impressed Current Systems: These require a bit more hands-on attention. Technicians need to regularly inspect the rectifier to make sure its voltage and amperage outputs are stable and exactly where they should be according to the design. They'll also hunt for any damaged cables or loose connections that could choke off the protective current.
The Shift to Proactive Remote Monitoring
In the old days, monitoring meant boots on the ground. Technicians would have to drive out to every single test station to manually take readings—a slow, expensive, and often inefficient process.
Today, technology has completely changed the game. Modern remote monitoring units (RMUs) are popping up everywhere, and for good reason. These small devices get installed at critical test points and beam performance data back to a central control room in real time. This means an operator can spot a rectifier failure or a sudden potential drop the moment it happens, dispatching a crew before any real damage can occur.
This isn't just about making life easier; it's also about meeting stricter rules. Regulatory bodies like the Canada Energy Regulator and the UK's Oil and Gas Authority are demanding more comprehensive corrosion management plans, which often includes remote monitoring. You can learn more about how these regulatory trends are shaping the industry.
This move represents a fundamental switch from a reactive to a proactive maintenance mindset. Instead of waiting for something to break, operators can now see problems developing and intervene early. This keeps the protective shield around the pipeline strong and unbroken, safeguarding the asset, the public, and the environment throughout its entire service life.
Common Questions About Cathodic Protection
Even after you get the hang of how cathodic protection works in theory, a lot of practical questions still come up. It's a cornerstone of modern infrastructure, but applying it in the real world can feel a bit tricky. Let's walk through some of the most common things people ask to clear up any confusion.
Getting these answers right is what bridges the gap between textbook knowledge and the day-to-day work of keeping a pipeline safe and operational.
How Long Does a Cathodic Protection System Last?
This is easily one of the most important questions, and the honest answer is: it depends entirely on the type of system you've got. A properly designed cathodic protection for pipes system is a long-term play, but its different parts wear out at different rates.
- Galvanic Systems: These systems are built around sacrificial anodes that, by design, get consumed over time. Depending on what they're made of, how big they are, and how corrosive the surrounding soil is, they typically last anywhere from 10 to 30 years. Once they're used up, you have to replace them to keep the protection going.
- Impressed Current Systems: These are built for the long haul. The inert anodes in an impressed current setup are often designed to last 20 to 40 years or more. The power supply components, like the rectifier, are the pieces that might need attention sooner, maybe every 15 to 25 years.
Ultimately, a smart initial design and consistent check-ups are the two biggest factors in getting the maximum life out of any system.
Can Cathodic Protection Be Used on Any Type of Pipe?
Not at all. Cathodic protection is a highly specialized tool meant for a specific job: protecting metallic pipes that can carry an electrical current. We're talking about materials like steel, cast iron, and ductile iron—the workhorses of the oil, gas, and water industries.
It’s completely useless for non-metallic pipes made from materials like PVC, HDPE, or fiberglass. These materials don't conduct electricity, so they aren't vulnerable to the electrochemical corrosion that cathodic protection is built to stop.
Do I Still Need a Coating with Cathodic Protection?
Yes, 100%. Thinking of it as an either/or decision is a big misunderstanding. Coatings and cathodic protection are a team, and they work far better together than they ever could alone.
A pipeline's coating is its first line of defense—a physical shield between the metal and the corrosive environment. Cathodic protection is the essential backup that protects the small, inevitable flaws like scratches and pinholes (what we call "holidays") that exist on every coated pipe.
Using both is, by far, the most effective and cost-efficient way to ensure a pipeline lasts for decades. The coating does the heavy lifting, which dramatically cuts down on the amount of electrical current the CP system needs to generate, making the whole operation more efficient.
What Are the Main Causes of System Failure?
Even the best-designed systems can run into trouble. Knowing what usually goes wrong is the key to good maintenance. Failures almost never happen suddenly; they’re usually the result of small problems that build up over time.
Here are the most common culprits we see:
- Physical Damage: This is a big one. Accidental digging from other construction work can easily sever cables or damage anodes.
- Anode Depletion: For galvanic systems, this is the most common "failure." The anodes simply did their job and need to be replaced.
- Power Supply Problems: In impressed current systems, a malfunctioning rectifier, a blown fuse, or a simple power outage will shut down protection immediately.
- Environmental Changes: New construction nearby might introduce stray electrical currents that interfere with your system. Even big changes in soil moisture can alter how much protection is needed.
This is exactly why routine monitoring isn't just a "good idea"—it’s a non-negotiable part of making sure a pipeline stays protected for its entire service life.
At Blue Gas Express, we know how critical it is to keep projects moving, especially when permanent gas lines are delayed. If utility timelines are holding up your construction or commissioning, our mobile CNG and LNG solutions deliver the immediate, reliable natural gas supply you need to stay on schedule. Don't let pipeline delays bring your progress to a halt—we can ensure a seamless transition with temporary gas services.
Learn how we can keep your project powered by visiting us at our website.